Molecular weight usually known as the molecular mass in current texts is an important factor when considering elements found in the periodic table. The molecular weight or mass of a molecule is said to be the mass of one of that molecule which is relative to the unified atomic mass un6.022 x 1023 molecules.it. The unified atomic mass unit is the number which is 1/12th of the mass of an isotope carbon 12. Molecular weight is very different from the molar mass which can be gotten or measured from the pack of molecules of the substance (therefore adding the isotopes) and shouldn’t be juxtaposed. Molecular mass cannot be used in stoichiometry because it does not deal with one molecule. Using molar mass in place of molecular mass will lead to errors in calculations.
The calculation of the molecular weight of a molecule can be done by the addition of all the molecular masses of the isotopes that makes up the molecule. The values of the isotope masses can be found on the periodic table. The molecular masses can be combined this way due to the relatively low binding energy that usually combines molecules and atoms which then leads to a minute change in mass. The change in mass can be equated to a change in energy with Einstein’s equation known as E=mc2. When the change is very negligible falling under the numbers from 10 to 9, the error in measurement from combining the isotope’s molecular masses is very little since they are joined with weak forces. It is important to note that this cannot be done with nuclear reaction, due to the high energy the reaction converts the mass to. The high energy will lead to a large error. The molecular weight can be expressed as the gram of the substance which is the macroscopic property to the molecules of the substance which is the microscopic property. So the unit for molecular weight is then given as gram per mole. Written as g/mol or kg/kmol.

Another way the molecular mass/weight of the molecule can be calculated is with the use of mass spectrometry. The molecules are separated into ions with the mass spectrometer, next the mass ratio to the charge of the ions is then measured. When using mass spectrometry the measured weight of the molecule is given as monoisotopic mass. That is, it is assumed that the molecular mass being measured contains the isotopes which are common and also the addition of all the atoms masses. Also, mass spectrometry when used on large molecules the average of the isotopes’ masses is used because the isotopes are of many different mix Monoisotopic masses cannot be found on the periodic table, they’re published elsewhere.
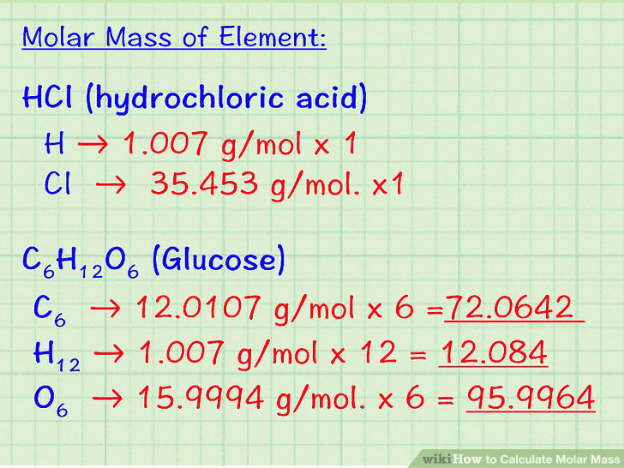
So to properly calculate the molecular weight or mass of a molecule, you first take in the amount of the atoms found in the molecule to be measured, then the atomic masses of the elements (found in the periodic table) contained in the molecule, add up the individual masses of the elements in the molecule multiplied by the previously calculated relative atomic mass. The molecular weight is then given.
To calculate accurately for the molecular weight of any substance it is best to try the online molecular weight calculator.